|
|
|
Copyright © 2001 by The Resilience Alliance
The following is the established format for referencing this article:
Kerr, J. T. 2001. Butterfly species richness patterns in Canada: energy, heterogeneity, and the potential consequences of climate change. Conservation Ecology 5(1): 10. [online] URL: http://www.consecol.org/vol5/iss1/art10/
A version of this article in which text, figures, tables, and appendices are separate files may be found by following this link.
Report, part of Special Feature on Pollinator Decline Butterfly Species Richness Patterns in Canada: Energy, Heterogeneity, and the Potential Consequences of Climate Change Jeremy T. Kerr
University of Oxford
- Abstract
- Introduction
- Climate Change and Butterfly Species Richness
- Current Species Richness Patterns among Canadian Butterflies
- Sampling Intensity and Spatial Autocorrelation
- Responses to this Article
- Acknowledgments
- Literature Cited
The distributions of most pollinator species are poorly documented despite their importance in providing ecosystem services. While these and other organisms are threatened by many aspects of the human enterprise, anthropogenic climate change is potentially the most severe threat to pollinator biodiversity. Mounting evidence demonstrates that there have already been biotic responses to the relatively small climate changes that have occurred this century. These include wholesale shifts of relatively well-documented butterfly and bird species in Europe and North America. Although studies of such phenomena are supported by circumstantial evidence, their findings are also consistent with predictions derived from current models of spatial patterns of species richness. Using new GIS methods that are highly precise and accurate, I document spatial patterns of Canadian butterfly diversity. These are strongly related to contemporary climate and particularly to potential evapotranspiration. An even more noteworthy finding is the fact that, for the first time, habitat heterogeneity, measured as the number of land cover types in each study unit, is proven to be an equally strong predictor of butterfly richness in a region where energy alone was thought to be the best predictor of diversity. Although previous studies reveal similar relationships between energy and diversity, they fail to detect the powerful link between richness and habitat heterogeneity. The butterflies of Canada provide a superb baseline for studying the effects of climate on contemporary patterns of species richness and comprise the only complete pollinator taxon for which this sort of analysis is currently possible.
KEY WORDS: butterflies, climate change, habitat heterogeneity, land cover, latitudinal gradients, pollinator, species richness, species richness-energy theory.
Published: April 5, 2001
Although pollinators undoubtedly play a key role in terrestrial ecosystems, distributions of most pollinator taxa remain poorly characterized. This phenomenon is common to many taxa but is particularly severe among invertebrates, including many pollinator taxa (Wilson 1992). The general public seems to have the impression that European honey bees (the introduced species, Apis mellifera) and bumble bees (Bombus spp.) are virtually the only pollinators (Buchmann and Nabhan 1996). The reality differs markedly. Thousands of species in North America contribute to pollination, a key ecosystem service (Daily 1997), including bats (Chiroptera), butterflies and moths (Lepidoptera), and particularly bees and wasps (Hymenoptera); flies such as Diptera (Kearns 2001) and beetles (Coleoptera) can also be pollinators. Declines in natural and honey-bee pollination rates may contribute to a pollination deficit in agricultural ecosystems, with serious consequences to food supply systems (Allen-Wardell et al. 1998, Kevan 2001).
Lepidopterans, particularly butterflies, are perhaps the best studied of all pollinator taxa, but are less important pollinators than bees. Available data about species distribution throughout much of North America are reliable, at least from a regional perspective, and may provide the basic information necessary to begin studying the effects of future human environmental disturbances. Surprisingly little has been done to document complete patterns of diversity even among butterflies, although many resources provide single-species distributions: see particularly http://www.npwrc.usgs.gov/resource/distr/lepid/bflyusa/bflyusa.htm and http://habanero.nhm.ukans.edu/SpeciesAnalyst/. Many taxonomic monographs for less well studied taxa include maps of species distributions based on various, but generally low, sampling intensities. Consequently, among complete pollinator taxa, large-scale diversity patterns in North America can most reliably be investigated for butterflies. Sampling intensity for butterflies throughout much of North America and Europe is comparatively high, aided by a considerable amateur interest in these species that is, perhaps, second only to that in birds.
In this study, I review the effects of climate change on pollinators and provide a first analysis of the biodiversity patterns for the butterflies of Canada, a complete pollinator taxon. A geographically broader analysis that includes several hundred lepidopterans, mostly moths, in Canada and the United States has been published elsewhere (Kerr et al. 1998). Although butterflies are a potentially useful flagship taxa among pollinators and for other species in general, surprisingly little has been done to characterize their diversity patterns using spatially explicit database technology. New analytical and remote sensing techniques, both statistical and technological, will help discover patterns that would be difficult to discern using older methods, and allow for much greater precision and accuracy.
The human influence on climate is surprisingly pervasive. For example, continental declines in precipitation in industrialized regions can be attributed to an increase in atmospheric particulate matter that inhibits raindrop nucleation (Rosenfeld 2000). Storm timing and frequency are influenced by industrial aerosols on the Atlantic seaboard of North America (Balling and Cerveny 1998). Most significantly, the global climate has been changing as a result of increasing greenhouse gas emissions since the beginning of the industrial revolution. The potential negative consequences of shifts in temperature, precipitation, and seasonality are sweeping and might easily become catastrophic over the next several decades. Many reviews of climate change theory and its possible biological consequences are available (Houghton et al. 1994, Bazzaz 1998).
Regional shifts in species distributions observed in Europe and North America offer strong circumstantial evidence that climate change is already affecting pollinator taxa. Recent evidence suggests that the northern distributional limits of many species in Europe have extended northward in conjunction with climate changes that took place during the 1900s (Parmesan 1996, Parmesan et al. 1999), a predictable result considering the climatic tolerances of these species (Kukal et al. 1991). Among 40 butterfly species used in the analysis of range retraction northward from their southern extents, only nine were found to have lost part of their southern ranges, while the southern ranges of most species have remained stable for the last 30–100 years. According to Parmesan et al. (1999), possible reasons for the low number of range retractions include the fact that (1) southern boundaries may be determined by interspecific interactions, (2) climatic factors in southern Europe and northern Africa have changed much less than in northern Europe, and (3) there are many potential refugia in regions corresponding to the southern limits of many species, such as the Pyrenees of southern France and northern Spain. The first two premises are strongly supported by experimental evidence from a few butterfly species (Kukal et al. 1991). Range retractions northward among species in Europe are to be expected if climate change is causing species ranges to shift, although other interpretations of Parmesan et al.'s (1999) results are possible. Although every effort has been made to eliminate from this study species whose ranges have shifted because of habitat alteration, this cannot be ruled out as an alternative explanation for at least some species (P. A. Opler, personal communication). This is always a problem with circumstantial evidence. However, the argument that climate change has already affected species ranges is considerably strengthened by complementary studies that demonstrate similar phenomena among birds (Thomas and Lennon 1999) and Euphydryas editha, a butterfly in the western USA (Parmesan 1996). Damage caused to biotic communities of nonpollinator taxa by climate change has also been documented. For example, precipitous declines in high-altitude amphibian communities in a Costa Rican cloud-forest habitat would appear to coincide with recent climatic changes (Pounds et al. 1999).
There is some early evidence that butterfly diversity in Canada is responding to climate changes that have occurred during the last few decades. At least two species, the Gorgone checkerspot (Chlosyne gorgone) and the Delaware skipper (Anatrytone logan), recently established breeding populations near Ottawa, Ontario, well beyond the previous northern limits of their respective ranges (P. W. Hall, personal communication). These butterflies are conspicuous, and their new localities are frequently surveyed by specialists, so there is little likelihood that these populations have been long established. Additional support for the circumstantial case that climatic changes have caused this range expansion northward is provided by the finding that a third species, the Tawny-edged skipper (Polites themistocles), from an area near Ottawa, now has a second generation during the longer warm periods in the region. These intriguing observations are consistent with other observations of range shifts from North America (Parmesan 1996), and with discoveries of extensive butterfly distribution shifts in Europe (Parmesan et al. 1999). Earlier studies of lepidopterans (Turner et al. 1987, Kerr et al. 1998) demonstrated that contemporary climate was important in determining spatial patterns of butterfly diversity in Canada, so there is reason to believe that further shifts in butterfly species distributions will occur because of the effects of climate change.
A few other studies document shifts in pollinator species ranges that can be attributed to anthropogenic climate change. Bryant et al. (1997) considered it likely that two nymphalid butterflies had shifted their ranges because of recent climate change, but most studies tend to focus on the anticipated biotic consequences of future changes (Sparks and Yates 1997). Few species or higher taxa, even in the UK where most taxa have been painstakingly documented, have been monitored over a long enough period or so intensively that observed range shifts can be attributed to recent climate changes. Changes in the distribution of a taxon are more often attributed to habitat loss, or perhaps to habitat fragmentation (Cane 2001). In most cases, this is probably the correct diagnosis (Swengel 1998a; Kerr et al. 2000). As climate change becomes increasingly obvious, it will more frequently be considered as a possible cause of shifts in the distribution of organisms (Pollard et al. 1996, Mikkola 1997, Tarrier and Leestmans 1997, Fleishman et al. 1998).
The direct effects of elevated atmospheric carbon dioxide concentrations on pollinators and their mutualistic plant hosts are also difficult to predict. Indirectly, elevated atmospheric CO2 is expected to modify ratios of carbon and nitrogen in plant tissues (Bazzaz 1998), possibly leading to changes in patterns of herbivory by organisms such as butterfly larvae (Rusterholz and Erhardt 1998). How this might affect communities of pollinators is uncertain. Furthermore, increasing concentrations of CO2 in the atmosphere will probably lead to changes in plant community structure, particularly in the proportions of C3 and C4 plants in a given habitat (Bazzaz 1998). It is too early to say whether these effects will influence the conservation status of particular pollinator species. However, stresses to ecosystems that are caused by climate change will act synergistically with other forms of human perturbation (Myers 1992), and the results of such synergisms cannot yet be predicted.
A thorough understanding of why species richness varies through space is useful when attempting to predict how it will respond to climate change or other perturbations (Kerr and Packer 1999). Thousands of studies have investigated the basis for spatial variation in species richness (Currie et al. 1999), but relatively few of these involve invertebrates (Kerr 1999), and an even smaller number focus on pollinator taxa (Kerr et al. 1998). The preponderance of empirical evidence suggests that regional variability in species richness is related to aspects of climatic energy (Wright 1983, Currie 1991, Wright et al. 1993), with additional influences of habitat heterogeneity (Kerr and Packer 1997, Fraser 1998). In general, climatic energy is able to explain 60–90% of the variability in species richness in cold and temperate areas, a finding of obvious importance in view of ongoing climatic change. Although many other hypotheses have been offered to explain regional patterns, there is not as much evidence to support them. An example is Rapoport's rescue hypothesis (Stevens 1989), but this is controversial and does not provide general explanations (Kerr 1999). Other hypotheses suggest that rates of evolution are higher in places with many species, thus accounting for regional variability, but there is virtually no evidence in support of this either (Cardillo 1999, Kerr and Currie 1999).
The predictability of biotic responses to climate change is probably scale dependent. Elaborate microcosm experiments (Davis et al. 1998), which are most convincing when they are supported by field observations (Brown et al. 1997), suggest that interspecific interactions, and perhaps even chance events, may play a substantial role in determining the habitat-level consequences of climatic alterations. Given the local scale at which biotic interactions (e.g., competition, predation) occur, this is only to be expected. However, these arguments, although they can be persuasive from a certain perspective, do not apply to large-scale predictions (Kerr and Packer 1998), nor are they supported by the substantial empirical record documenting species range shifts in conjunction with shifting climatic zones (Graham et al. 1996). Although it may be possible to predict the effects of climate change on the numbers of species in a region ( Bazzaz 1998, Kerr and Packer 1998), it will be much harder to predict how individual species may respond; this will require detailed study of butterfly communities across geographical gradients (Swengel 1998b).
This analysis of butterfly diversity patterns in Canada is based on the full Canadian National Collection (CNC) database. The CNC collection contains more than 110,000 records of 292 butterfly and skipper species recorded during the 1900s in Canada (Layberry et al. 1998). Records are stored according to precise latitudinal and longitudinal coordinates. There are a number of advantages to a database of this kind. First, collection intensity is high, so the patterns that emerge from an analysis of all species records are robust. Second, the distributions of the constituent species are not interpolated to produce continuous range maps. This is commonly done in the production of range maps for taxa across large geographic ranges and creates significant problems of increased spatial autocorrelation for the purposes of analysis. Spatial autocorrelation is discussed in greater detail below.
MethodsThree different quadrat systems were constructed for the analysis of butterfly diversity across the complete, extensive terrestrial area of Canada. Two of these systems were equal in area and extended over two and five degrees of latitude, respectively, with variable longitudinal extent because degrees of longitude cover progressively less distance toward the poles. The third system was a digital version of a commonly used system (Currie and Paquin 1987, Kerr and Packer 1997) consisting of quadrats that are 2.5° x 2.5° south of 50° latitude and 2.5° latitude x 5° longitude north of this mark. Although the area varies among the quadrats in Currie's grid system, repeated studies (Currie 1991, Kerr et al. 1998) have failed to find any indication that these small variations in quadrat area have a significant effect on the diversity of any vertebrate, plant, or invertebrate taxa. The C++ programs (AT&T C++, version 2.1) used to build these systems and other software developed for this project are available free of charge from the authour or from the Spatial Ecology of Canadian Biodiversity link at http://mathbiol.zoo.ox.ac.uk/jeremy/.
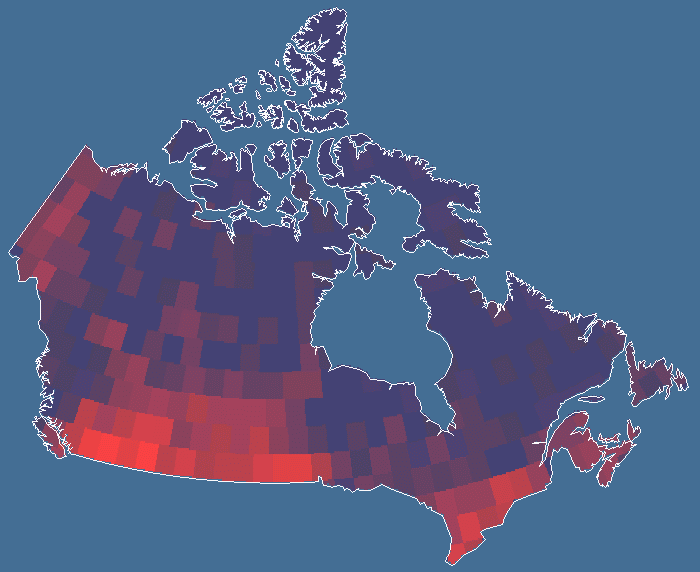
By means of an additional program, the number of species occurring in each of the quadrats in all three quadrat systems was tallied to produce the database for butterfly species diversity across Canada. Species records that occurred more than once in the same quadrat, either because a species was recorded more than once in the same location over different years or because the same species was recorded in two locales within one quadrat, were eliminated. Consequently, no species was recorded more than once per quadrat. The resulting diversity values were assigned to each quadrat in the respective quadrat systems in Idrisi 32 for Windows (Fig. 1). The total number of records per quadrat was tallied for the largest (5°) and smallest (2°) quadrat systems, and these values were regressed against species richness. This provided a measurement of sampling intensity that was unbiased with respect to actual diversity per quadrat. Residual values were assigned to the quadrats in Idrisi 32 for visual inspection of a sampling intensity map.
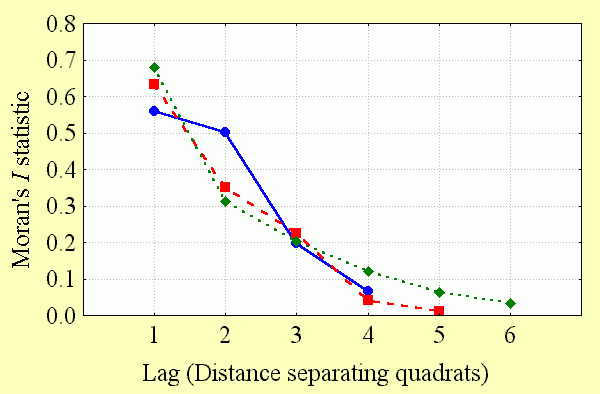
The extent of spatial autocorrelation in the data was measured by calculating a series of Moran's I values with increasing lag. This method tests for spatial autocorrelation between sampling quadrats as the distance between them increases from adjacent (lag = 1) to the point at which the significance of the Moran's I statistic declines to zero (lag > 1). The plot of Moran's I statistics against lag distance is known as a correlogram and is presented here for each of the quadrat systems used in this study (Fig. 2). Spatial autocorrelation beyond the distance where the Moran's I statistic became nonsignificant was not measured, because there was no plausible biological reason why there should be negative spatial autocorrelation over large geographical gradients in this analysis (Kerr et al. 2000).
Each sampling grid was superimposed over digital climatic maps using Idrisi 32 for Windows; the environmental predictors used are summarized in Table 1. For each quadrat in these grids, the annual mean, minimum and maximum values were extracted for each of the study's climate variables. These included actual and potential evpotranspiration, temperature, primary productivity as described in the Chikugo model (Uchijima and Seino 1985), and precipitation (all at 30-min resolution). Net primary productivity was included because it is a method of measuring the amount of energy available for use by the biota and because it is itself derived from other measurements of climate.
|
Table 1. Summary of factors hypothesized to determine regional patterns of species richness. Although all of these factors were examined in this study, the focus was on the two that provide notably powerful statistical results: land cover variability and potential evapotranspiration.
2J. S. Olson, J. Watts, and L. Allison. Carbon in Live Vegetation of Major World Ecosystems. (Oak Ridge: Oak Ridge National Laboratory, 1983). |
Similar analyses were performed for measurements of habitat heterogeneity based on a digital elevation model at 5-min resolution, Holdridge data sets (Leemans 1990), Olson biome maps at 30-min resolution (Olson et al. 1983), and remotely sensed land cover data based on 8-km AVHRR satellite data (DeFries et al. 1998). Aspects of elevation included relief (the difference between maximum and minimum elevations), mean, minimum, and maximum elevations. Measurements of biome and land cover variability included a simple count of the number of different biome or land cover types per quadrat, respectively.
Bivariate plots of all hypothesized environmental factors were constructed with butterfly species richness for each of the three study grid systems. Square root transformations were used to adjust for minor problems with heteroscedastic residuals when these occurred. Linear regression or, for nonlinear relationships, polynomial regression analyses were used to further explore the relationships between contemporary environment and butterfly richness. A multiple regression model to predict butterfly richness in each of the grid systems was constructed using a combination of standard forward and backward stepwise regression techniques (Zar 1984).
Results and discussionPatterns of butterfly species richness discovered here were found to be similar to those of many other taxa studied using coarser data, including Cicindelidae (Kerr and Currie 1999), mammals (Kerr and Packer 1997), and birds (Currie 1991). Butterfly diversity generally declines to the north and east but is highest in southern Ontario and the south-central Great Plains. In both regions, there are areas of relatively low diversity. Diversity is lowest in the eastern arctic and higher in the heterogeneous western arctic. Many of the species found in these regions are most broadly distributed in the United States and have only a small proportion of their ranges in Canada. The most parsimonious explanation for the similarity among the species richness patterns of so many taxa in this geographical area is that the same factors control these patterns (Currie et al. 1999).
Butterfly diversity increases with high regional habitat heterogeneity (land cover variation) and climatic energy (potential evapotranspiration). These factors explain most of the variation in butterfly richness (50–80%) seen in Fig. 3, Fig. 4, and Table 2. However, the predictability of these regional scale models declines with quadrat size. There are a number of possible reasons for this. Although butterfly data are among the best available for any taxon, perhaps second only to those available for birds, data sampling intensity is not equally strong everywhere, as explained below. The number of records of butterfly species per quadrat necessarily declines with quadrat size. At finer spatial scales, there is progressively less potential for variation between adjacent quadrats, reducing the detectability of geographical gradients of diversity. Ecological factors, chance, and anthropogenic modifications to the environment all play a role in determining whether butterfly populations can be found within individual habitats, and these factors are more likely to have a quantitative effect as quadrat size declines.
|
Table 2. Multiple regression models incorporating land cover variability and potential evapotranspiration. Butterfly richness is square-root transformed in all models. Potential evapotranspiration and land cover variation are comparably strong at all scales in these analyses. Factor p refers to the significance level of the individual variable within the regression model, whereas model p refers to the significance level of the model itself.
| ||||||||||||||||||||||||||||||||||||||||||||
If the correlations reported here between contemporary climate and habitat heterogeneity are causative, climate change is likely to have significant effects on diversity patterns on a regional scale. These effects will operate directly through changes in aspects of climate, particularly those that are heat-related, and through changes in habitat diversity. Earlier conceptual models suggest that regional-scale habitat diversity will decline in a rapidly warming climate (Peters and Darling 1985). Observational results for high-altitude communities of desert mammals support this model (McDonald and Brown 1992), although these are disputed (Skaggs and Boecklen 1996); additional support is provided by studies of Euphydryas editha butterfly communities (Parmesan 1996) and European butterflies (Parmesan et al. 1999). Easterling et al. (2000) summarize some major biotic consequences of climate change, underscoring the essentially unpredictable nature of climatic changes at local spatial scales. Long-term geological evidence suggests that mass extinctions may be caused by unexpected variations in annual climate, primarily because of a lack of physiological tolerance for unusually cold conditions (Ivany et al. 2000)
Although spurious patterns of biological diversity often emerge because of enormous variations in sampling intensity in different geographic locations, this phenomenon is unlikely to have a qualitative influence on the patterns observed in this study. To test this proposition, the relationships between butterfly species richness and land cover variability and between species richness and potential evapotranspiration were examined. If sampling intensity was, in fact, a major cause of the diversity patterns observed, these relationships should have been strong. Instead, the relationship between sampling intensity, as measured by the number of records for all species per quadrat, and potential evapotranspiration was relatively weak, as was the relationship between sampling intensity and land cover variability per quadrat (R2 = 0.19–0.34). Correlations between butterfly richness and energy and between richness and heterogeneity were about twice as strong at all spatial scales. Furthermore, patterns of Canadian butterfly diversity were consistent at different spatial scales: those observed in the 2° quadrat system strongly resembled those from the variable area and 5° systems, respectively. Variation in sampling intensity is a likely source of "noise" in the relationships between richness and environmental factors that inversely relates to quadrat size, providing one explanation for the decline in the coefficient of determination of regression models with decreasing quadrat size.
Spatial autocorrelation is significant among butterfly diversity observations and declines at a similar rate with increasing distance between observations at all spatial scales (Fig. 4). Unlike the unusual result reported for GAP data for Wyoming vertebrates (Fraser 1998), spatial autocorrelation is still significant at larger spatial scales (5° quadrats). This makes sense: results suggesting that diversity values in adjacent sampling locations do not covary are contradictory to the results widely observed at many spatial scales (Legendre and Legendre 1998: 8).
Most large-scale studies of species diversity patterns have relied on interpolated data that increase spatial autocorrelation and numerous "false presence" values for species in a quadrat (Adams and Woodward 1989). Knowledge of the distribution of a species over geographical ranges consists of the sum of the sampling locales at which its presence has been recorded. These sampling locales are often joined together to form a continuous species distribution map, e.g., http://woodland.bio.ic.ac.uk/research/tigerb/rangepaper.htm. Although the study species may not actually be found in the areas in between sampling points, it will nevertheless be recorded as present. As a result, such highly interpolated data will not only contain many errors compared to a well-sampled point map but also inflate problems with spatial autocorrelation by creating smoothed diversity patterns (Williams and Gaston 1996).
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a comment, follow this link. To read comments already accepted, follow this link.
I am happy to thank NSERC for the postdoctoral support that made this research possible. Conversations with and comments from Bob May, Dick Southwood, David Currie, Brad Hawkins, and Tony Francis improved this manuscript considerably. Two anonymous reviewers provided additional thoughtful criticisms that have also helped this work. I am grateful to Jim Cane for his help throughout the editorial process.
Adams, J. M., and F. I. Woodward. 1989. Patterns in tree species richness as a test of the glacial extinction hypothesis. Nature 339: 699-701.
Allen-Wardell, G., P. Bernhardt, R. Bitner, A. Burquez, S. Buchmann, J. Cane, P. A. Cox, V. Dalton, P. Feinsinger, D. Inouye, M. Ingram, C. E. Jones, K. Kennedy, P. Kevan, H. Koopowitz, R. Medellin, S. Medellin-Morales, G. P. Nabhan, B. Pavlik, V. Tepedino, P. Torchio, and S. Walker. 1998. The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conservation Biology 12: 8-17.
Balling, R. C., and R. S. Cerveny. 1998. Weekly cycles of air pollutants, precipitation and tropical cyclones in the coastal NW Atlantic region. Nature 394: 561-563.
Bazzaz, F. A. 1998. Tropical forests in a future climate: changes in the biological diversity and impact on the global carbon cycle. Climatic Change 39: 317-336.
Brown, J. H., T. J. Valone, and C. G. Curtin. 1997. Reorganization of an arid ecosystem in response to recent climate change. Proceedings of the National Academy of Science (USA) 94: 9729-9733.
Bryant, S. R., C. D. Thomas, and J. S. Bale. 1997. Nettle-feeding nymphalid butterflies: temperature, development and distribution. Ecological Entomology 22: 390-398.
Buchmann, S. L., and G. P. Nabhan. 1996. The forgotten pollinators. Island Press, Washington, D.C., USA.
Cane, J. H. 2001. Habitat fragmentation and native bees: a premature verdict? Conservation Ecology 5(1): 3 [online] URL: http://www.consecol.org/vol5/iss1/art3.
Cardillo, M. 1999. Latitude and rates of diversification in birds and butterflies. Proceedings of the Royal Society of London (B) 266: 1221-1225.
Currie, D. J. 1991. Energy and large-scale patterns of animal and plant species richness. American Naturalist 137: 27-49.
Currie, D. J., and V. Paquin. 1987. Large-scale biogeographic patterns of species richness of trees. Nature 329: 326-327.
Currie, D. J., J. T. Kerr, and A. P. Francis. 1999. Some general propositions about the study of spatial patterns of species richness. Ecoscience 6: 392-399.
Daily, G. C. 1997. Nature's services: societal dependence on natural ecosystems. Island Press, Washington, D.C., USA.
Davis, A. J., L. S. Jenkinson, J. H. Lawton, B. Shorrocks, and S. Wood. 1998. Making mistakes when predicting shifts in species range in response to global warming. Nature 391: 783-785.
DeFries, R. S., M. Hansen, J. R. G. Townshend, and R. Sohlberg. 1998. Global land cover classifications at 8 km spatial resolution: the use of training data derived from Landsat imagery in decision tree classifiers. International Journal of Remote Sensing 19: 3141-3168.
Easterling, D. R., G. A. Meehl, C. Parmesan, S. A. Changnon, T. R. Karl, and L. O. Mearns. 2000. Climate extremes: observations, modeling, and impacts. Science 289: 2068-2074.
Fleishman, E. G., T. Austin, and A. D. Weiss. 1998. An empirical test of Rapoport's rule: elevational gradients in montane butterfly communities. Ecology 79: 2482-2493.
Fraser, R. H. 1998. Vertebrate species richness at the mesoscale: relative roles of energy and heterogeneity. Global Ecology and Biogeography 7: 215-220.
Graham, R. W., E. L. Lundelius Jr., M. A. Graham, E. K. Schroeder, R. S. Toomey III, E. Anderson, A. D. Barnosky, J. A. Burns, C. S. Churcher, D. K. Grayson, R. D. Guthrie, C. R. Harington, G. T. Jefferson, L. D. Martin, H. G. McDonald, R. E Morlan, H. A. Semken Jr., S. D. Webb, L. Werdelin, and M. C. Wilson. 1996. Spatial response of mammals to late Quaternary environmental fluctuations. Science 272: 1601-1606.
Houghton, J. T., L. G. Meira Filho, J. Bruce, L. Hoesung, B. A. Callander, E. Haites, N. Harris, and K. Maskell. 1994. Climate change 1994: radiative forcing of climate change and an evaluation of the IPCC IS92 Emission Scenarios, published for the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press, Cambridge, UK.
Ivany, L. C., W. P. Patterson, and K. C. Lohmann. 2000. Cooler winters as a possible cause of mass extinctions at the Eocene/Oligocene boundary. Nature 407: 887-890.
Kearns, C. A. 2001. North American Dipteran pollinators: assessing their value and conservation status. Conservation Ecology 5(1): 5 [online] URL: http://www.consecol.org/vol5/iss1/art5.
Kerr, J. T. 1999. Weak links: "Rapoport's rule" and large-scale species richness patterns. Global Ecology and Biogeography 8: 47-54.
Kerr, J. T., and D. J. Currie. 1999. The relative importance of evolutionary and environmental controls on broad scale patterns of species richness in North America. Ecoscience 6: 329-337.
Kerr, J. T., and L. Packer. 1997. Habitat heterogeneity as a determinant of mammal species richness in high energy regions. Nature 385: 252-254.
Kerr, J. T., and L. Packer. 1998. The impact of climate change on mammal diversity in Canada. Environmental Modeling and Assessment 49: 263-270.
Kerr, J. T., and L. Packer. 1999. Epicauta species richness patterns in North America: the importance of energy. Biodiversity and Conservation 8: 617-628.
Kerr, J. T., A. Sugar, and L. Packer. 2000. Indicator taxa, rapid biodiversity assessment, and nestedness in an endangered ecosystem. Conservation Biology 14: 1726-1734.
Kerr, J. T., R. L. Vincent, and D. J. Currie. 1998. Determinants of Lepidoptera richness in North America. Ecoscience 5: 448-453.
Kevan, P. G., and T. Phillips. 2001. The economics of pollinator declines: assessing the consequences. Conservation Ecology 5(1): 8 [online] URL: http://www.consecol.org/vol5/iss1/art8.
Kukal, O., M. P. Ayres, and J. M. Scriber. 1991. Cold tolerance of the pupae in relation to the distribution of swallowtail butterflies. Canadian Journal of Zoology 69: 3028-3037.
Layberry, R. A., P. W. Hall, and D. J. Lafontaine. 1998. The butterflies of Canada. University of Toronto Press, Toronto, Canada.
Leemans, R. 1990. Global data sets collected and compiled by the Biosphere Project: working paper. International Institute for Applied Systems Analysis, Laxenburg, Austria.
Legendre, P., and L. Legendre. 1998. Numerical ecology. Second English edition. Elsevier, Amsterdam, the Netherlands.
McDonald, K. A., and J. H. Brown. 1992. Using montane mammals to model extinctions due to global change. Conservation Biology 6: 409-415.
Mikkola, K. 1997. Population trends of Finnish Lepidoptera during 1961–1996. Entomologica Fennica 8: 121-143.
Myers, N. 1992. Synergisms: joint effects of climate change and other forms of habitat destruction. Pages 344-354 in R. L. Peters and T. E. Lovejoy, editors. Global warming and biological diversity. Yale University Press, New Haven, Connecticut, USA.
Olson, J. S., J. Watts, and L. Allison. 1983. Carbon in live vegetation of major world ecosystems. U.S. Department of Energy Publication Number W-7405-ENG-26. Oak Ridge National Laboratory, Oak Ridge, Tennessee, USA.
Parmesan, C. 1996. Climate and species' range. Nature 382: 765-766.
Parmesan, C., N. Ryrholm, C. Steganescu, J. K. Hill, C. D. Thomas, H. Descimon, B. Huntley, L. Kaila, J. Kullberg, T. Tammaru, W. J. Tennent, J. A. Thomas, and M. Warren. 1999. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399: 579-583.
Peters, R. L., and J. D. Darling. 1985. The greenhouse effect and nature reserves. BioScience 35: 707-717.
Pollard, E., P. Rothery, and T. J. Yates. 1996. Annual growth rates in newly established populations of the butterfly Pararge aegeria. Ecological Entomology 21: 365-369.
Pounds, J. A., M. P. L. Fogden, and J. H. Campbell. 1999. Biological response to climate change on a tropical mountain. Nature 398: 611-615.
Rosenfeld, D. 2000. Suppression of rain and snow by urban and industrial air pollution. Science 287: 1793-1796.
Rusterholz, H. P., and A. Erhardt. 1998. Effects of elevated CO2 on flowering phenology and nectar production of nectar plants important for butterflies of calcareous grasslands. Oecologia 113: 341-349.
Skaggs, R. W., and W. J. Boecklen. 1996. Extinctions of montane mammals reconsidered: putting a global-warming scenario on ice. Biodiversity and Conservation 5: 759-778.
Sparks, T. H., and T. J. Yates. 1997. The effect of spring temperature on the appearance dates of British butterflies, 1883–1993. Ecography 20: 368-374.
Stevens, G. C. 1989. The latitudinal gradient in geographical range: how so many species coexist in the tropics. American Naturalist 133: 240-256.
Swengel, A. B. 1998a. Effects of management on butterfly abundance in tallgrass prairie and pine barrens. Biological Conservation 83: 77-89.
Swengel, A. B. 1998b. Comparisons of butterfly richness and abundance measures in prairie and barrens. Biodiversity and Conservation 7: 1639-1659.
Tarrier, M., and R. Leestmans. 1997. Losses and acquisitions probably linked to the effects of global climatic warming on western Mediterranean lepidopteran fauna (Lepidoptera, Papilionoidea). Linneana Belgica 16: 23-36.
Thomas, C. D., and J. J. Lennon. 1999. Birds extend their ranges northwards. Nature 399: 213.
Turner, J. R. G., C. M. Gatehouse, and C. A. Corey. 1987. Does solar energy control organic diversity? Butterflies, moths and the British climate. Oikos 48: 195-205.
Uchijima, Z., and H. Seino. 1985. Agroclimatic evaluation of net primary productivity of natural vegetation: Chikugo model for evaluating net primary productivity. Journal of Agriculture and Meteorology 40: 343-352.
Williams, P. H., and K. J. Gaston. 1998. Biodiversity indicators: graphical tecniques, smoothing, and searching for what makes relationships work. Ecography 21: 551-560.
Wilson, E. O. 1992. The diversity of life. Harvard University Press, Cambridge, Massachusetts, USA.
Wright, D. H. 1983. Species-energy theory: an extension of species-area theory. Oikos 41: 496-506.
Wright, D. H., D. J. Currie, and B. A. Maurer. 1993. Energy supply and patterns of species richness on local and regional scales. Pages 66-74 in R. E. Ricklefs and D. Schluter, editors. Species diversity in ecological communities. University of Chicago Press, Chicago, Illinois, USA.
Zar, J. H. 1984. Biostatistical analysis. Prentice-Hall, Toronto, Canada.
Address of Correspondent:
Jeremy T. Kerr
Environmental Monitoring Section
Canada Centre for Remote Sensing
588 Booth Street
Ottawa, Ontario, Canada K1A 0Y7
Phone: (613) 947-1266
Fax: (613) 947-1406
Jeremy.Kerr@CCRS.NRCan.gc.ca