|
|
|
Copyright ©1998 by The Resilience Alliance*
Rod Bhar and Lenore Fahrig. 1998. Local vs. landscape effects of woody field borders as barriers to crop pest movement. Conservation Ecology [online] 2(2): 3. Available from the Internet. URL: http://www.consecol.org/vol2/iss2/art3/
A version of this article in which text, figures, tables, and appendices are separate files may be found by following this link.
Research Local vs. Landscape Effects of Woody Field Borders as Barriers to Crop Pest Movement Rod Bhar1 and Lenore Fahrig2
1Ottawa-Carleton Institute of Biology; 2Carleton University
- Abstract
- Introduction
- Hypotheses
- The Model
- Simulation Experiment
- Results
- Discussion
- Conclusion
- Responses
- Acknowledgments
- Literature Cited
Maintenance of woody borders surrounding crop fields is desirable for biodiversity conservation. However, for crop pest management, the desirability of woody borders depends on the trade-off between their effects at the local field scale and the landscape scale. At the local scale, woody borders can reduce pest populations by increasing predation rates, but they can also increase pest populations by providing complementary habitats and reducing movement rate of pests out of crop fields. At the regional scale, woody borders can reduce pest populations by reducing colonization of newly planted crop fields. Our objective was to develop guidelines for maximizing pest control while maintaining woody borders in the landscape. We wished to determine the conditions under which the regional effect of borders on colonization can outweigh local enhancement effects of borders on pest populations. We built a stochastic, individual-based, spatially implicit simulation model of a specialist insect population in a landscape divided into a number of crop fields. We conducted simulations to determine the conditions under which woody borders enhance vs. reduce the regional pest population size. The following factors were considered: landscape fragmentation, crop rotation period, barrier effect of woody borders, disperser success rate, and effect of woody borders on local survival. The simulation results suggest that woody borders are most likely to enhance regional control of crop pests if (1) the woody borders are very effective in reducing insect movement from one crop field to another, and (2) crop rotation is on a very short cycle. Based on these results, our preliminary recommendations are that woody borders should contain dense, tall vegetation to reduce insect movement, and crops should be rotated on as short a cycle as possible. These conditions should ensure that woody borders can be maintained for their conservation value without enhancing crop pest populations. The results are encouraging because the two most important factors are not sensitive to details of pest habitat use; the recommendations should apply across most pest species.
KEY WORDS: biodiversity, crop pest, crop rotation, dispersal, fencerow, field margin, hedgerow, patchy population, pest control, shelterbelt, simulation model, woody border.
The increasing scale of agricultural activity over the past several decades has resulted in destruction of strips of wooded vegetation bordering crop fields, variously termed "fencerows," "shelterbelts," or "hedgerows" (Medley et al. 1995). This loss is of concern because of the ecological benefits commonly attributed to woody borders, including erosion control, conservation of biodiversity, and pest control.
Woody borders provide habitat for many organisms that would otherwise not be found in agricultural areas, including various plants (Baudry 1988, Marshall 1988, Jobin et al. 1996), mammals (Ogilvie and Furman 1959, Pollard and Relton 1970, Eldridge 1972, Yahner 1983), and birds (Arnold 1983, Osborne 1984, Green et al. 1994, Parish et al. 1994, 1995, MacDonald and Johnson 1995, Sparks et al. 1996, Fuller et al. 1997). Although less well demonstrated, woody borders are also thought to provide dispersal routes for organisms moving between patches of remnant forest, thus contributing to the recolonization and/or rescue of local populations (Wegner and Merriam 1979, Fahrig and Merriam 1985, Johnson and Adkisson 1985, Merriam and Lanoue 1990, Bennett et al. 1994).
The effects of woody borders on farmland insect communities are complex. Most studies have focused on woody borders as habitat for predaceous insects, primarily carabid beetles and spiders (Sotherton 1985, Wratten 1988, Sustek 1992, Charrier et al. 1997). It is generally assumed that woody borders have a negative effect on pest insects in crop fields because they enhance predator populations. However, most studies on predators in woody borders simply document the densities of predators in borders vs. crop fields, and do not demonstrate a net beneficial effect of woody borders in reducing pest populations.
Studies comparing overall insect diversity (not just of predatory species) in woody borders vs. neighboring crop fields have generally found much higher diversity in woody borders (Lewis 1969a, Bowden and Dean 1977, Dennis and Fry 1992). Fahrig and Jonsen (1998) found correlations between density and diversity within several groups of agricultural insects. Together, these observations suggest that woody borders may enhance both herbivore and predator populations. There are two possible mechanisms for this enhancement. First, woody borders can represent complementary habitat (sensu Dunning et al. 1992) for crop insects, providing overwintering sites (Dennis and Fry 1992), summer aestivation sites (Manglitz 1958), mating sites (Hawkes 1973), or foraging sites (Hawkes 1973, Bowden and Dean 1977). When pesticides are in use, woody borders may represent a refuge habitat for insects (Powell 1986, Dyer and Landis 1997).
Second, woody borders can enhance pest populations by acting as barriers to movement of insects out of crop fields, thus trapping them within the fields and promoting local population build-up. Several studies have shown that movement rates of specialist insects are greatly reduced on encountering a border between host plants and non-host plants, and that this effect increases with increasing height of the non-host vegetation (Lawrence 1982, Capinera et al. 1985, Power 1987, Bohlen and Barrett 1990, Frampton et al. 1995, Holmes and Barrett 1997). Woody borders have been shown to slow or stop movement of various insects from neighboring crop fields and/or from more distant points in the landscape (Lewis 1969b, Hawkes 1973, Bowden and Dean 1977, Fry 1994, Mauremooto et al. 1995).
On a larger scale, however, such barriers to movement may reduce regional populations of crop insects by limiting movement between crop fields. The agricultural landscape is a dynamic mosaic of patches; the crop type grown in a given field changes over time due to crop rotation. Therefore, regional persistence and abundance of crop insects depends on their between-field movement for colonization of newly planted fields of host plants (Levins 1969, Sherratt and Jepson 1993, Weisz et al. 1994). On a regional scale, woody borders may decrease populations by limiting colonization of newly planted fields.
In summary, maintenance of woody borders is desirable for biodiversity conservation. However, for insect pest management, the desirability of woody borders depends on the trade-off between their negative effects on pest populations (increased predation, decreased colonization of new crop fields) and their positive effects on pest populations (complementary habitat, movement barrier resulting in local population build-up).
Objective: Maximizing the Regional Benefits of Woody BordersIn this study, we were concerned with the situation in which woody borders enhance pest populations locally within fields. Our goal was to determine how local enhancement of pests might best be countered by the negative effects of borders on regional populations of insect pests. In other words, under what conditions does the regional negative effect outweigh the local positive effect? We express the results as recommendations for ensuring that woody borders can both maintain biodiversity and control pests at a regional scale.
We identified attributes of pest biology, local habitat, and landscape structure, which we hypothesized should determine the trade-off between positive local effects and the negative regional effect of woody borders on pest population density. We then developed a simulation model of the population dynamics of a crop pest in a patchy landscape, and conducted simulation experiments to (1) check the logic of a set of verbal hypotheses and (2) rank the relative importance of the potential effects. From this ranking, we made recommendations for land use practices that enhance the negative effects of woody borders on crop pest populations, while maintaining their contribution to biodiversity conservation.
1) Landscape fragmentation. Woody borders are more likely to reduce the regional pest population when the total area of the host crop in the landscape is more fragmented, i.e., when there are more and smaller crop fields.
For a given crop rotation period, more crop fields will reach the end of their life-spans (i.e., the fields will be converted to some other crop) in any given year in a more fragmented than in a less fragmented landscape. If we assume that the total crop area is identical in both cases, then there are more new (but smaller) crop fields planted each year in a more fragmented landscape. To maximize its regional population, the pest must colonize all new fields quickly. It will take longer to colonize a large number of smaller fields than a smaller number of large fields. Because woody borders interfere with dispersal and, hence, reduce colonization, woody borders should be more likely to reduce the regional pest population when the landscape is more fragmented.
2) Crop rotation period. Woody borders are more likely to reduce the regional population if the period of crop rotation is short.
As in hypothesis 1, assume that, for every crop field that is ploughed under and planted in some other crop, and is thereby removed from the landscape from the crop pest's point of view, a new field of the host crop is planted. To maximize the regional pest population, dispersers must colonize the new field. The shorter the duration of crop fields, the more often new fields will be created and the more often new fields must be colonized. Because woody borders interfere with dispersal and colonization, woody borders are more likely to reduce the regional population when field duration (crop rotation cycle) is short.
Dispersal and Survival3) Barrier effect. Woody borders are more likely to reduce the regional pest population when their effect as a barrier to interfield movement is stronger.
The greater the barrier effect of a woody border, the more it will reduce dispersal and, thus, colonization of new fields. The positive local effects of woody borders on the pest are then likely to be outweighed by the reduction in colonization. In addition, when a field reaches the end of its life-span and is planted in a different (non-host) crop, the specialist pest insects must leave the field if they are to survive. A woody border that acts as a strong barrier will cause more of these insects to remain in the old field and die.
4) Dispersal success rate. Woody borders are more likely to reduce the regional pest population when the dispersal success rate is low.
Dispersal success rate is the probability that an individual, having left its field, will find its way to another suitable field. In this study, individuals that do not successfully disperse are assumed to die. The lower the dispersal success rate, the greater the likelihood that the regional population will go extinct. When the dispersal success rate is low, anything that further reduces dispersal, such as woody borders, is more likely to reduce the regional population than to increase it. Hypotheses 3 and 4 are closely related, but different. Hypothesis 3 suggests that woody borders hindering emigration will reduce the regional population. Hypothesis 4 suggests that this effect will be increased if the mortality rate of dispersers that do leave is high.
5) Woody borders and survival. Woody borders are more likely to reduce the regional population when the increase in survival rate due to woody borders is small.
We have argued that woody borders can provide complementary or refuge habitat for pest species, thus increasing their survival rate. At the regional scale, this increase in survival is offset by a decrease in colonization of new fields because of the barrier effect of the woody borders. When the positive effect of woody borders on survival is weak, the balance will be in favor of the barrier effect, thus reducing the regional pest population.
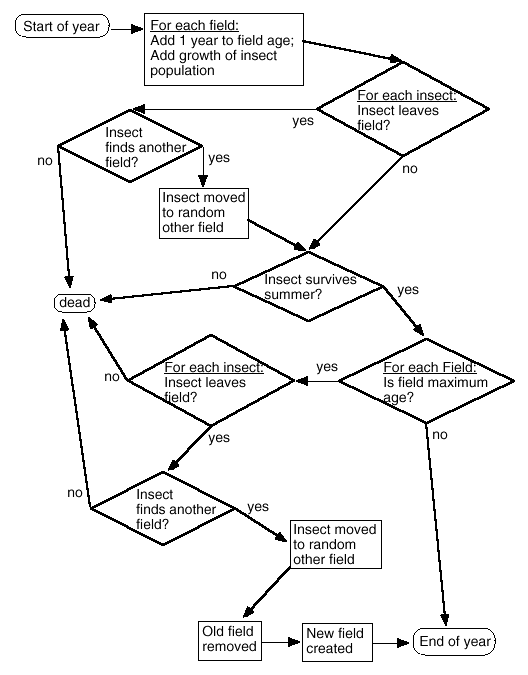
We built a stochastic, individual-based simulation model (Fig.1) of a specialist insect population in a landscape divided into a number of crop fields. Only fields containing the particular crop on which the insect specializes ("host crop fields") are included in the simulations.
Fig. 1. Flow chart of the simulation model.
|
Runs of the model were done in pairs (termed a "run pair"), once for a landscape with woody field borders and again for the same landscape without woody field borders. All parameter values were kept constant within each run pair. The capacity of the landscape was set at 1000 insects, where it remained throughout the runs. Each field in the landscape had a capacity equal to the total capacity (1000) divided by the number of fields. That is, all fields within a run pair were assumed to be the same size. To minimize the simulation time required, each run began at the capacity, with a regional population of 1000 individuals, equally divided among the fields. At the start of each run pair, parameter values were selected randomly from within set ranges.
The number of host crop fields (FIELDS) was selected between 2 and 20. The maximum field age (time of conversion to another crop: MAXAGE) defines the period of crop rotation, and was selected between 2 and 10 years. At the beginning of each run, the fields were assigned starting ages ranging from 1 year up to MAXAGE, in an even distribution, to ensure that the runs did not begin with fields that were all the same age.
The crop insect had one generation per year, with reproduction occurring in the early spring and dispersal occurring after reproduction, in late spring. Mortality occurred both during dispersal and during the summer. In the fall, any field that had reached MAXAGE was ploughed and seeded in a different crop (i.e., removed from the simulation). The insects then either dispersed from the old field or died, and a new host crop field was seeded for the next spring. Colonization of the new field could occur in the following year, after reproduction.
There were four stages modeled in each year: early spring, late spring, summer, and fall. In early spring, the population in each field was increased by a fixed proportion, reflecting the net effect of births in spring and deaths of adults during the previous winter. The proportional increase (R ) was a random number between 0.1 and 10 per year, chosen at the beginning of each run pair; it remained at that level throughout the run pair. After the growth phase, the population in each field was checked against the field capacity and was reduced to the capacity if that was exceeded.
Dispersal from fields was implemented in late spring as follows. Before the beginning of the run pair, two random numbers were selected between 0 and 1. The larger random number was the probability of an individual leaving a field in the landscape that had no woody borders (DISP). The smaller number was the probability of an individual leaving a field in the landscape that had woody borders (DISPWOOD). A third random number between 0 and 1 was selected at the beginning of the run pair, to represent the probability of a dispersing individual finding another field (FIND). During the run, for each individual in each time step, a random number was chosen between 0 and 1. If this number was less than or equal to the probability of an individual leaving a field (DISP or DISPWOOD, depending on whether the landscape had woody borders), the individual left its field. For individuals that left their field, another random number between 0 and 1 was chosen. If this number was less than or equal to FIND, then the individual was placed randomly within one of the other fields. Individuals that left their field and did not find another field were assumed to die.
During the summer, the probability of survival of the insect depended on the degree to which woody borders enhanced survival (through their function as complementary or refuge habitat). At the beginning of a run pair, two random numbers between 0 and 1 were chosen. The smaller number was the probability of an individual surviving the summer in a field in the landscape with no woody borders (SURV), and the larger number was the probability of an individual surviving the summer in a field in the landscape with woody borders (SURVWOOD). Note again that, in this study, we did not consider the case in which woody borders actually reduce local pest survival. Each summer, for each individual, a random number between 0 and 1 was chosen. If this number was less than or equal to the survival probability (SURV or SURVWOOD, depending on whether or not the landscape had woody borders), the individual survived the summer.
Crop rotation occurred in the fall. Any field that had reached MAXAGE was removed from the run (it was ploughed and seeded in a different crop). Pest individuals could leave the field at this time, depending on DISP or DISPWOOD (whichever applied). The likelihood that an individual leaving the field would find another field depended again on FIND. Any individual that did not leave the old (ploughed) field and find another field died. For each old field removed in the fall, a new field was added in the following spring.
The simulation experiment was designed to (1) check the logic of the hypotheses and (2) rank the relative importance of the hypothesized factors in determining the effect of woody borders on a regional crop pest population. We conducted10,000 run pairs (20,000 runs).
Each run pair lasted for 100 years, after which the final regional populations in the two runs (with and without woody borders) were compared. If the landscape with woody borders had a larger final population, a result of 0 was recorded. If the landscape without woody borders had a larger final population, a result of 1 was recorded. Therefore, a 1 indicates that the woody borders had a net positive effect on pest control, i.e., a negative effect on the pest population. If the population in one landscape went extinct before the end of the run, then the other landscape was recorded as having the higher final population. If the populations in both landscapes went extinct, then the one that went extinct last was recorded as having the larger final population.
At the beginning of each run pair, a random value was selected for each of the parameters, as previously described: FIELDS (2-20), MAXAGE (2-10), R (0.1-10), DISP (larger of two random numbers 0-1), DISPWOOD (smaller of the same two random numbers 0-1), FIND (0-1), SURV (smaller of two random numbers 0-1), and SURVWOOD (larger of the same two random numbers 0-1).
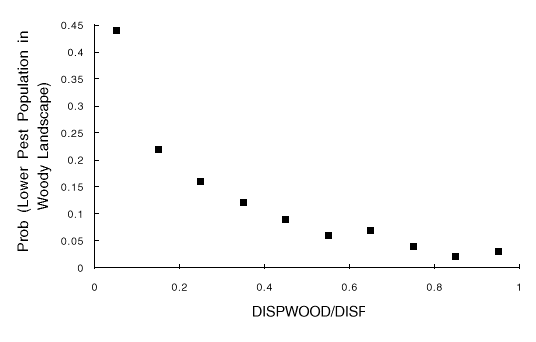
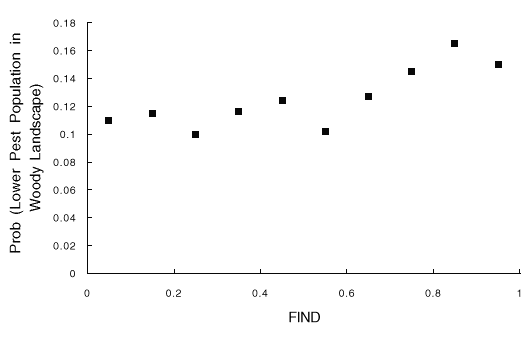
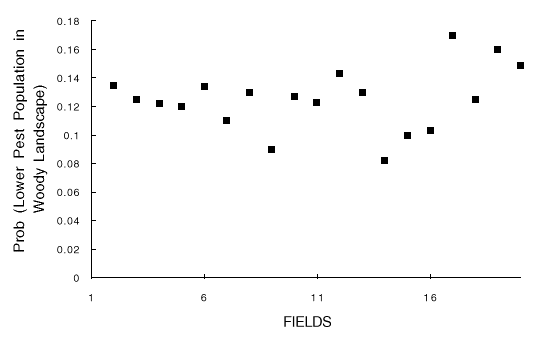
Hypotheses 1 and 2 were examined by varying FIELDS and MAXAGE, respectively. Hypothesis 3 was examined by the effect of DISPWOOD/DISP, the degree to which woody borders reduce dispersal. Hypotheses 4 was examined by varying FIND. Hypothesis 5 was examined by the effect of SURVWOOD/SURV, the degree to which woody borders enhance survival of the pest population.
The simulation results were analyzed using logistic regression analysis, in which the response variable was 0 or 1, representing the cases in which woody borders enhanced or depressed the regional pest population, respectively. The predictor variables were FIELDS, MAXAGE, DISPWOOD/DISP, FIND, and SURVWOOD/SURV.
The landscape with woody borders had a lower regional pest population size than the landscape without woody borders in 1,230 of the 10,000 run pairs. In the remaining 8,770 run pairs, the landscape with woody borders had the higher regional pest populations. Results of the logistic regression analysis are shown in Table 1, and the relationships between the predictor variables and the degree of enhancement of pest control by woody borders are shown in Figs. 2-6.
Table 1. Multiple logistic regression analysis of simulation results. The response variable is ln(p/(1-p)), where p is the probability that the landscape with woody borders has a smaller pest population (better pest control) than the landscape without woody borders. We conducted 10,000 simulation run pairs (with and without woody borders). Random values for each predictor variable were selected at the beginning of each run, as described in the text. Variables are ranked in decreasing magnitude of effect. All coefficients are significant (alpha = 0.05), except the one for FIELDS.
|
The logic imbedded in hypotheses 2, 3, and 5 was confirmed in the simulations. The result for hypothesis 4, the effect of disperser success (FIND), was opposite to that predicted, and hypothesis 5, the expected effect of field number (FIELDS), was not observed. The factors can be ranked from highest to lowest importance (Table 1) as DISPWOOD/DISP, MAXAGE, SURVWOOD/SURV, FIND, and FIELDS (no significant effect), corresponding to hypotheses 3, 2, 5, inverse of 4, and 1, respectively.
The simulation results suggest that woody borders are most likely to enhance regional control of crop pests if (1) the woody borders are very effective in reducing insect movement out of crop fields (DISPWOOD/DISP), and (2) crop rotation is on a very short cycle (MAXAGE). Based on these results, our preliminary recommendations are that woody borders should contain dense, tall vegetation to reduce insect movement, and crops should be rotated on as short a cycle as possible.
Because well-vegetated borders are likely to harbor a higher diversity of organisms (Green et al. 1994, Parish et al. 1994), the first recommendation is complementary to the role of woody borders in conservation of biodiversity. This recommendation is also consistent with the suggestion that predator populations are most enhanced in stable, vegetationally diverse borders (Topping and Sunderland 1994, Freemark and Boutin 1995).
To test these predictions would require very large-scale (multilandscape) observational studies comparing crop insects in landscapes with and without woody borders, and having short vs. long crop rotation periods. Most studies of farmland insects have been conducted at the scale of a few to a few hundred meters. This is due to the difficult logistics and high cost of large-scale studies, and to the current emphasis in agroecology on experimental work. Well-planned, large-scale observational studies would be required to test our predictions.
Hypothesis 1, the expected positive effect of field number or landscape fragmentation (FIELDS) on improving pest control in landscapes with woody borders, was not observed in the simulation study. Note that "fragmentation" here refers literally to "breaking apart" of habitat, sensu Fahrig (1998). This result, combined with the importance of frequent crop rotation, is consistent with the general finding that, in dynamic landscapes, resident populations are much more affected by the rate of change of landscape pattern than by the pattern itself (Fahrig 1991, 1992, 1998). However, when woody borders are present, a more fragmented agroecosystem, containing larger numbers of smaller fields, will have a greater total length of woody border, which will enhance biodiversity (see Introduction ).
The third most important factor determining the net effect of woody borders was the degree to which woody borders increase survival of insect pests by providing complementary or refuge habitat (SURVWOOD/SURV). Although this factor was fairly important, it does not lend itself well to general management recommendations. For a suite of crop pests, the woody border is likely to provide different kinds of complementary habitat for different species. We cannot envision a single design of woody border that would simultaneously eliminate such complementary habitats while maintaining high biodiversity of woody borders.
The result for FIND predicts that the degree to which woody borders enhance crop pest control should increase with increasing probability that insects leaving a field will find another suitable field before dying (disperser success) (Fig. 5). It seems that increasing disperser success increases the need for the dispersal barrier (woody borders) or, conversely, that decreasing disperser success decreases the effectiveness of woody borders as barriers to colonization of new fields. In an agroecosystem, dispersal success will depend on the survival of insects moving through non-host areas such as other crops, forests, wetlands, or urban areas. Disperser success will often depend on species-specific responses to various habitat types. We cannot envision recommendations for land use practices that would simultaneously improve disperser success for all dispersing crop insects. A possible exception is that dispersal success of most crop insects may be low if there is heavy pesticide use on crop fields through which the dispersers must travel to reach another host crop field. Our simulations predict that such application of pesticides would reduce the negative effect of woody borders on regional pest populations. In any event, FIND is much less important than the first two factors (DISPWOOD/DISP and MAXAGE), implying that attempts to manipulate disperser success are not likely to have a large impact on the effectiveness of woody borders for pest control.
Model AssumptionsWe used an individual-based formulation to reduce the number of parameters needed. Each process in a stochastic, population-based model requires at least two parameters, one for the central tendency and one for the variability of the random variable. For example, implementation of dispersal in a stochastic population model requires values for the mean dispersal rate and variance of the dispersal rate. In contrast, a stochastic, individual-based model requires only a single parameter value, the probability of dispersal, which is applied separately to each individual in the population.
Although it was developed for a specific purpose, our model has some similarities with other, more general patchy-population models. Its structure is intermediate between the "BIDE"- type models (Birth+Immigration-Death-Emigration; reviewed in Pulliam 1988), and spatially explicit patchy-population models (e.g., Fahrig 1992). Like the BIDE models, ours is not spatially explicit. The relatively high turnover of fields suggests that a spatially explicit formulation is not necessary (Fahrig 1992, 1998). Unlike BIDE and like Fahrig (1992), our model explicitly includes disperser mortality. Dispersers typically have higher mortality rates than residents (e.g., Steen 1994, Van Vuren and Armitage 1994), and Ruxton et al. (1997a,b ) have shown that inclusion of disperser mortality in patchy-population models has important effects on predicted local and regional population dynamics.
Our goal was to examine the barrier effects of woody borders on crop pest populations. There are two hypothesized counteracting effects: the local effect, which traps pests in a field and leads to enhanced pest populations; and the regional effect, in which limited movement between fields leads to lowered regional populations. We simplified the study by not including local pest control by predator populations in woody borders. Addition of this effect would have greatly increased the number of run pairs in which the landscape with woody borders produced lower pest populations than the landscape without woody borders. However, the ranking of the factors examined here would not have been altered. Therefore, the main land use recommendations, for dense, tall fencerows and frequent crop rotation, would not have been altered.
Although pesticide use was not explicitly included in the model, it was implicitly included in two parameters. First, improved survival of crop insects in the woody borders (SURVWOOD/SURV) could be because they represent a refuge habitat from pesticides used on the crop field. Secondly, low values of disperser success (FIND) could be due to use of pesticides on crop fields through which dispersing insects must travel in order to find another host crop field.
For most insects, there is very little, if any, empirical information that could be used to set the parameter values in our model. However, this is not a concern in our simulation study, because our purpose was not to simulate a particular insect in a particular landscape, but rather to develop general rules. The ranges selected for the parameters were purposefully wide, to avoid the possibility that conclusions about relative importance of the different factors depended on parameter ranges. All probability values were selected over the full range of 0-1. The range of MAXAGE (2-10) was most restricted. Allowing MAXAGE=1 would increase the importance of this already very important parameter. Given the model structure, MAXAGE=1 was not possible because all the insects on the landscape would die out at the end of the first year. Such an extremely dynamic landscape is also rather unrealistic; however, our results suggest that it would undoubtedly be very effective for pest control in a landscape with woody borders. The largest value of MAXAGE (10) represents quite a stable agricultural landscape. Expanding MAXAGE beyond 10 years could increase its relative importance by only a small amount (Fig. 3).
The range for number of fields (FIELDS) (2-20) was purposefully wide. A value of one field was not possible, because this would have resulted in complete disappearance of the population when that field reached MAXAGE. Because there was no effect of FIELDS over the range 2-20, it seems unlikely that further fragmentation of the landscape would produce an effect.
In our simulation study, we did not incorporate any correlations between parameters, but varied them independently. Several such correlations are possible, depending on the particular insects and landscapes one may have in mind. For example, the rate of dispersal from fields (DISP or DISPWOOD) may be higher when fields are smaller (larger value of FIELDS) (Kareiva 1985, Bach 1988, Sheehan and Shelton 1989). Dispersal rate and survival rate (SURV or SURVWOOD) may be negatively correlated (Harrington and Taylor 1990). Immigration rate and survival rate may be positively correlated (Paradis 1995). Depending on the movement behavior of the insect, its ability to find new fields (FIND) may depend on field size (Fahrig and Paloheimo 1987, Sheehan and Shelton 1989, Capman et al. 1990), which is controlled by FIELDS. However, because it is not known whether any of these correlations is general across insects and landscapes, we did not include them in our simulations.
Based on our simulations, we suggest the following preliminary land management recommendations for enhancing regional crop pest control while maintaining woody borders in the landscape: (1) woody borders should be designed to restrict insect movement as much as possible, i.e., they should contain dense, tall vegetation; and (2) crop rotation should occur on as short a cycle as possible. At this stage, the results are simply predictions arising from our model assumptions. Currently, there is insufficient information to evaluate the generality of the assumptions. The results are nevertheless encouraging, because the two most important factors are not sensitive to details of pest habitat use; the recommendations should apply across most pest species. Such generality is needed, because we can not simultaneously tailor a landscape for the specific attributes of each of hundreds of different pest species.
The results also suggest the need for larger scale studies of pest populations in agroecosystems (Schettini 1992). Insect populations, particularly pest species, disperse widely and are typically not localized to single crop fields. Effects of features such as woody borders may be very different at the local scale than at the regional scale. Effects of land use decisions at the regional scale must therefore be studied at that scale. Because controlled field experiments typically are not possible at a regional scale, progress in this area will most likely be made through a combination of computer modeling and large-scale sampling programs.
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a comment, follow this link. To read comments already accepted, follow this link.
Acknowledgments
We thank Darren Bender, Lutz Tischendorf, and John Wegner for helpful suggestions on an earlier draft. Two anonymous reviewers also provided helpful suggestions. This research was supported by a Natural Sciences and Engineering Research Council of Canada grant to L. Fahrig and by Carleton University.
Arnold, G. W. 1983. The influence of ditch and hedgerow structure, length of hedgerows, and area of woodland and garden on bird numbers on farmland. Journal of Applied Ecology 20: 731-750.
Bach, C. E. 1988. Effects of host plant patch size on herbivore density: underlying mechanisms. Ecology 69: 1103-1117.
Baudry, J. 1988. Hedgerows and hedgerow networks as wildlife habitat in agricultural landscapes. Pages 111-124 in J. R. Park, editor. Environmental management in agriculture. Belhaven Press, London, UK.
Bennett, A. F., K. Henein, and G. Merriam. 1994. Corridor use and the elements of corridor quality: chipmunks and fencerows in a farmland mosaic. Biological Conservation 68: 155-165.
Bohlen, P. J., and G. W. Barrett. 1990. Dispersal of the Japanese beetle (Coleoptera: Scarabaeidae) in strip-cropped soybean agroecosystems. Environmental Entomology 19: 955-960.
Bowden, J., and G. J. W. Dean. 1977. The distribution of flying insects in and near a tall hedgerow. Journal of Applied Ecology 14: 343-354.
Capinera, J. L., T. J. Weissling, and E. E. Schweizer. 1985. Compatibility of intercropping with mechanized agriculture: effects of strip intercropping of pinto beans and sweet corn on insect abundance in Colorado. Journal of Economic Entomology 78: 354-357.
Capman, W. C., G. O. Batzli, and L. E. Simms.1990. Responses of the common sooty wing skipper to patches of host plants. Ecology 71: 1430-1440.
Charrier, S., S. Petit, and F. Burel. 1997. Movements of Abax parallelepipedus (Coleoptera: Carabidae) in woody habitats of a hedgerow network landscape: a radio-tracing study. Agriculture, Ecosystems and Environment 61: 133-144.
Dennis, P., and G. L. A. Fry. 1992. Field margins: can they enhance natural enemy population densities and general arthropod diversity on farmland? Agriculture, Ecosystems and Environment 40: 95-115.
Dunning, J. B., B. J. Danielson, and H. R. Pulliam. 1992. Ecological processes that affect populations in complex landscapes. Oikos 65: 169-175.
Dyer, L. E., and D. A. Landis. 1997. Influence of noncrop habitats on the distribution of Eriborus terebrans (Hymenoptera: Ichneumonidae) in cornfields. Environmental Entomology 26: 924-932.
Eldridge, J. 1972. Some observations on the dispersion of small mammals in hedgerows. Journal of Zoology 165: 530-534.
Fahrig, L. 1991. Simulation methods for developing general landscape-level hypotheses of single-species dynamics. Pages 417-442 in M. G. Turner and R. H. Gardner, editors. Quantitative methods in landscape ecology. Springer-Verlag, New York, New York, USA.
_______ . 1992. Relative importance of spatial and temporal scales in a patchy environment. Theoretical Population Biology 41: 300-314.
_______ . 1998. When does fragmentation of breeding habitat affect population survival? Ecological Modelling 105: 273-292.
Fahrig, L., and I. Jonsen. 1998. Effect of habitat patch characteristics on abundance and diversity of insects in an agricultural landscape. Ecosystems 1: 197-205.
Fahrig, L., and G. Merriam. 1985. Habitat patch connectivity and population survival. Ecology 66: 1762-1768.
Fahrig, L., and J. Paloheimo. 1987. Interpatch dispersal of the cabbage butterfly. Canadian Journal of Zoology 65: 616-622.
Frampton, G. K., T. Cilgi, G. L. A. Fry, and S. D. Wratten. 1995. Effects of grassy banks on the dispersal of some carabid beetles (Coleoptera: Carabidae) on farmland. Biological Conservation 71: 347-355.
Freemark, K., and C. Boutin. 1995. Impacts of agricultural herbicide use on terrestrial wildlife in temperate landscapes: A review with special reference to North America. Agriculture, Ecosystems and Environment 52: 67-91.
Fry, G. L. A. 1994. The role of field margins in the landscape. Pages 31-40 in Field margins: integrating agriculture and conservation. BCPC Monograph Number 58.
Fuller, R. J., R. J. Trevelyan, and R. W. Hudson. 1997. Landscape composition models for breeding bird populations in lowland English farmland over a 20 year period. Ecography 20: 295-307.
Green, R. E., P. E. Osborne, and E. J. Sears. 1994. The distribution of passerine birds in hedgerows during the breeding season in relation to characteristics of the hedgerow and adjacent farmland. Journal of Applied Ecology 31: 677-692.
Harrington, R., and L. R. Taylor. 1990. Migration for survival: fine-scale population redistribution in an aphid, Myzus persicae. Journal of Animal Ecology 59: 1177-1193.
Hawkes, C. 1973. Factors affecting the aggregation of the adult cabbage root fly (Erioischia bouché ) at hedges. Journal of Applied Ecology 10: 695-703.
Holmes, D. M., and G. W. Barrett. 1997. Japanese beetle (Popillia japonica ) dispersal behavior in intercropped vs. monoculture soybean agroecosystems. American Midland Naturalist 137: 312-319.
Jobin, B., C. Boutin, and J.-L. DesGranges. 1996. Habitats fauniques du milieu rural quebecois: une analyse floristique. Canadian Journal of Botany 74: 323-336.
Johnson, W. C., and C. S. Adkisson. 1985. Dispersal of beech nuts by blue jays in fragmented landscapes. American Midland Naturalist 113: 319-324.
Kareiva, P. 1985. Finding and losing host plants by Phyllotreta: patch size and surrounding habitat. Ecology 66: 1809-1816.
Lawrence, W. S. 1982. Sexual dimorphism in between and within patch movements of a monophagous insect: Tetraopes (Coleoptera: Cerambycidae). Oecologia 53: 245-250.
Levins, R. 1969. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bulletin of the Entomolgical Society of America 15: 237-240.
Lewis, T. 1969a. The diversity of the insect fauna in a hedgerow and neighbouring fields. Journal of Applied Ecology 6: 453-458.
_______ . 1969b. The distribution of flying insects near a low hedgerow. Journal of Applied Ecology 6: 443-452.
MacDonald, D. W., and P. J. Johnson. 1995. The relationship between bird distribution and the botanical and structural characteristics of hedges. Journal of Applied Ecology 32: 492-505.
Manglitz, G. R. 1958. Aestivation of the alfalfa weevil. Journal of Economic Entomology 51: 506-508.
Marshall, E. J. P. 1988. The dispersal of plants from field margins. Pages 136-143 in J. R. Park, editor. Environmental management in agriculture. Belhaven Press, London, UK.
Mauremooto, J. R., S. D. Wratten, S. P. Worner, and G. L. A. Fry. 1995. Permeability of hedgerows to predatory carabid beetles. Agriculture, Ecosystem and Environment 52: 141-148.
Medley, K. E., B. W. Okey, G. W. Barrett, M. F. Lucas, and W. H. Renwick. 1995. Landscape change with agricultural intensification in a rural watershed, southwestern Ohio, U.S.A. Landscape Ecology 10: 161-176.
Merriam, G., and A. Lanoue. 1990. Corridor use by small mammals: field measurements for three experimental types of Peromyscus leucopus. Landscape Ecology 4: 123-131.
Ogilvie, R. T., and T. Furman. 1959. Effect of vegetational cover of fence rows on small mammal populations. Ecology 40: 140-141.
Osborne, P. 1984. Bird numbers and habitat characteristics in farmland hedgerows. Journal of Applied Ecology 21: 63-82.
Paradis, E. 1995. Survival, immigration and habitat quality in the Mediterranean pine vole. Journal of Animal Ecology 64: 579-591.
Parish, T., K. H. Lakhani, and T. H. Sparks. 1994. Modelling the relationship between bird population variables and hedgerow and other field margin attributes. I. Species richness of winter, summer and breeding birds. Journal of Applied Ecology 31: 764-775.
Parish, T., K. H. Lakhani, and T. H. Sparks. 1995. Modelling the relationship between bird population variables and hedgerow and other field margin attributes. II. Abundance of individual species and of groups of similar species. Journal of Applied Ecology 32: 362-371.
Pollard, E., and J. Relton. 1970. Hedges. V. A study of small mammals in hedges and cultivated fields. Journal of Applied Ecology 7: 549-557.
Powell, W. 1986. Enhancing parasitoid activity in crops. Pages 319-340 in J. Waage and D. Greathead, editors. Insect parasitoids. Academic Press, London, UK.
Power, A. G. 1987. Plant community diversity, herbivore movement, and an insect-transmitted disease of maize. Ecology 68: 1658-1669.
Pulliam, H.R. 1988. Sources, sinks, and population regulation. American Naturalist 132: 652-661.
Ruxton, G., J. L. Gonzalez-Andujar, and J. N. Perry. 1997a. Mortality during dispersal stabilizes local population fluctuations. Journal of Animal Ecology 66: 289-292.
Ruxton, G., J. L. Gonzalez-Andujar, and J. N. Perry. 1997b. Mortality during dispersal and the stability of a metapopulation. Journal of Theoretical Biology 186: 389-396.
Schettini, T.M. 1992. Multilevel habitat management as a paradigm for developing regenerative crop production systems. HortScience 27: 736-737.
Sheehan, W., and A. M. Shelton. 1989. Parasitoid response to concentration of herbivore food plants: finding and leaving plants. Ecology 70: 993-998.
Sherratt, T. N., and P. C. Jepson. 1993. A metapopulation approach to modelling the long-term impact of pesticides on invertebrates. Journal of Applied Ecology 30: 696-705.
Sotherton, N. W. 1985. The distribution and abundance of predatory Coleoptera overwintering in field boundaries. Annals of Applied Biology 106: 17-21.
Sparks, T.H., T. Parish, and S. A. Hinsley. 1996. Breeding birds in field boundaries in an agricultural landscape. Agriculture, Ecosystems and Environment 60: 1-8.
Steen, H. 1994. Low survival of long distance dispersers of the root vole (Microtus oeconomus ) Annales Zoologica Fennici 31: 271-274.
Sustek, Z. 1992. Windbreaks and line communities as migration corridors for carabids (Col. Carabidae) in the agricultural landscape of South Moravia. Ekológia 11: 259-271.
Topping, C. J., and K. D. Sunderland. 1994. A spatial population dynamics model for Lepthyphantes tenuis (Araneae: Linyphiidae) with some simulations of the spatial and temporal effects of farming operations and land-use. Agriculture, Ecosystems and Environment 48: 203-217.
Van Vuren, D., and K. B. Armitage. 1994. Survival of dispersing and philopatric yellow-bellied marmots: what is the cost of dispersal? Oikos 69: 179-181.
Wegner, J. F., and G. Merriam. 1979. Movements by birds and small mammals between a wood and adjoining farmland habitats. Journal of Applied Ecology 16: 349-357.
Weisz, R., Z. Smilowitz, and B. Christ. 1994. Distance, rotation, and border crops affect Colorado potato beetle (Coleoptera: Chrysomelidae) colonization and population density and early blight (Alternaria solani ) severity in rotated potato fields. Journal of Economic Entomology 87: 723-729.
Wratten, S. D. 1988. The role of field boundaries as reservoirs of beneficial insects. Pages 144-150 in J. R. Park, editor. Environmental management in agriculture. Belhaven Press, New York, New York, USA.
Yahner, R. H. 1983. Population dynamics of small mammals in farmstead shelterbelts. Journal of Mammalogy 64: 380-386.
Address of Correspondent:
Lenore Fahrig
Ottawa-Carleton Institute of Biology
Carleton University
1125 Colonel By Drive
Ottawa, ON K1S 5B6
Phone: 613-520-3856
Fax: 613-520-4497
lfahrig@ccs.carleton.ca
*The copyright to this article passed from the Ecological Society of America to the Resilience Alliance on 1 January 2000.